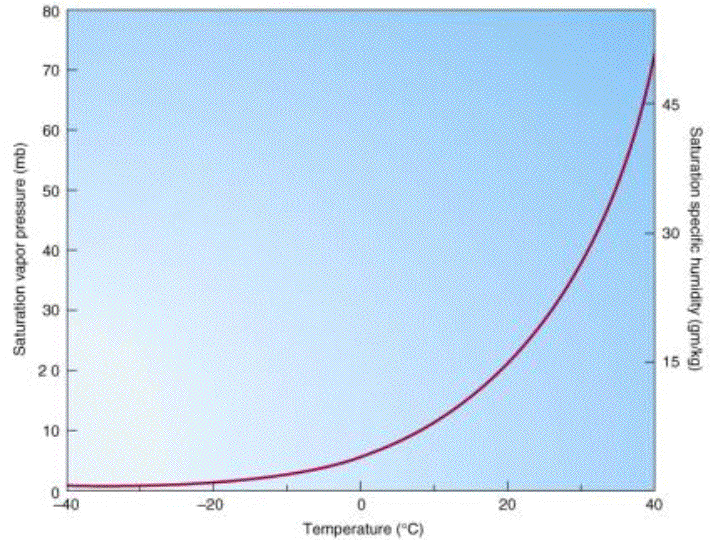
As
air becomes cooler, its capacity to hold water in vapor form decreases significantly. This is known as the Clausius-Clapeyron relationship. This means that if air containing water vapor cools
down, it will
eventually become saturated with water vapor. As the
air cools further,
enough water vapor condenses
into droplets to maintain the air at its saturation point.